Kāpae
Ua ʻike lōʻihi nā lāʻau lapaʻau Incretin e hoʻomaikaʻi i nā mea ʻeluaka mālama ʻana i ka glucose kokoahoemi kino kino. ʻO nā lāʻau lapaʻau incretin kuʻuna ke kuhikuhi mua i kaʻO ka mea loaʻa GLP-1, oiaiTirzepatidehōʻike i kahi hanauna hou o "twincretin” nā ʻelele - e hana anaʻelua GIP (glucose-dependent insulinotropic polypeptide)aGLP-1nā mea hoʻokipa.
Ua hōʻike ʻia kēia hana ʻelua i ka hoʻomaikaʻi ʻana i nā pono metabolic a hoʻoikaika i ka pohō kaumaha ʻoi aku ka nui ma mua o GLP-1 agonists wale nō.
ʻO ka SURMOUNT-1 Study Design
PALAPALA-1he arandomized, pālua-makapō, pae 3 hoʻokolohua lapaʻauhana ʻia ma nā kahua he 119 ma ʻeiwa mau ʻāina.
ʻO nā poʻe i komo pū me nā mākua i:
- Obese(BMI ≥ 30), a i ʻole
- Ke kaumaha nui(BMI ≥ 27) me hoʻokahi maʻi maʻi pili i ke kaumaha (e laʻa, hypertension, dyslipidemia, sleep apnea, a i ʻole ka maʻi cardiovascular).
ʻO ka poʻe me ka maʻi maʻi maʻi, ka hoʻohana ʻana i ka lāʻau hōʻemi kaumaha, a i ʻole ke ʻoki ʻana i ka bariatric ma mua i kāpae ʻia.
Ua hoʻokaʻawale ʻia nā mea komo e loaʻa i hoʻokahi pule i hoʻokahi pule o:
- Tirzepatide 5 mg, 10 mg, 15 mg, a i ʻole
- Placebo
Ua loaʻa pū i nā mea komo a pau ke alakaʻi alakaʻi ola:
- A caloric deficit o 500 kcal / lā
- Mea iki loa150 mau minuke o ka hana kino i kēlā me kēia pule
Ua hoʻomau ka mālama ʻana72 pule, me a20-week dose-escalation phaseukali ʻia e kahi manawa mālama 52-wiki.
Hōʻike Hōʻike
He huina o2,359 i komoua kākau inoa ʻia.
ʻO ka makahiki maʻamau44.9 makahiki, 67.5% he mau wahine, me ka meanke kaumaha o ke kino o 104.8 kgaBMI o 38.0.
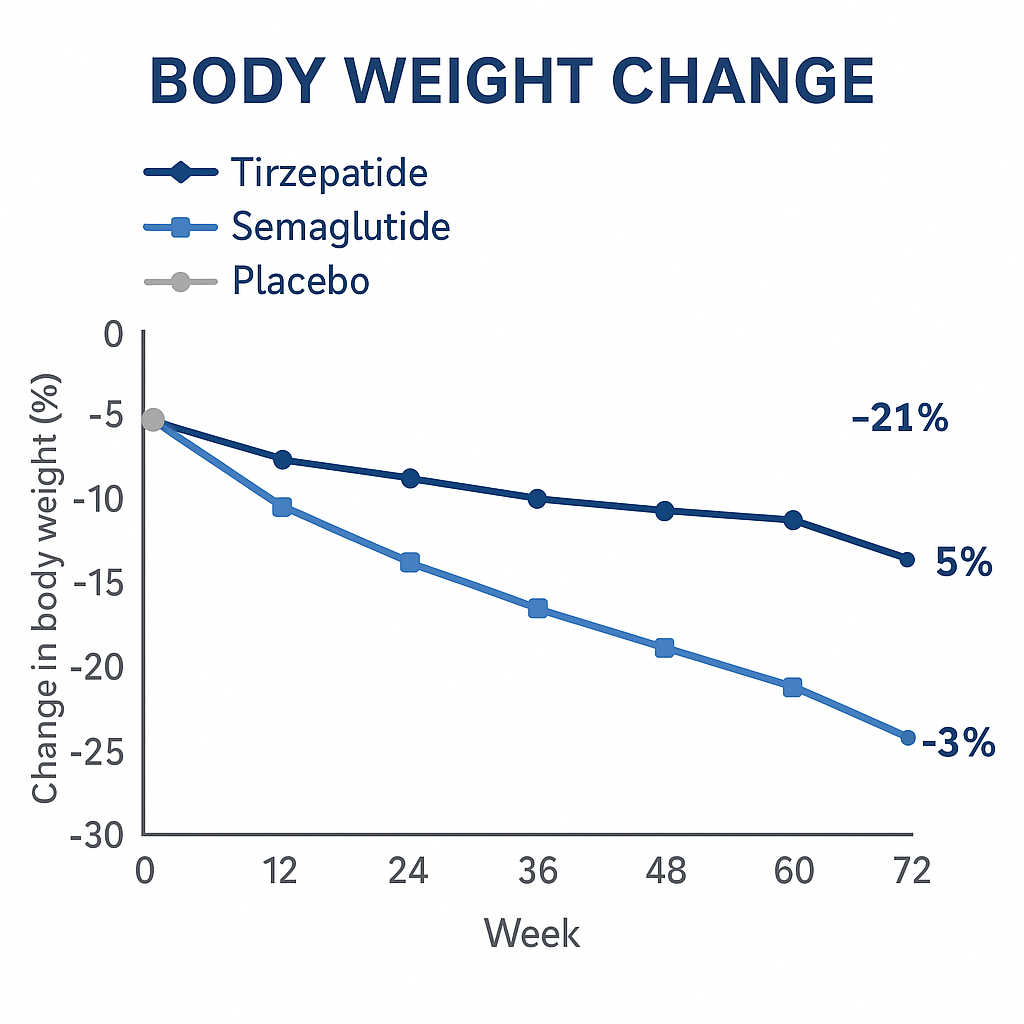
ʻO ka hoemi ʻana i ke kaumaha o ke kino ma ka pule 72
| Pūʻulu Dose | % Hoololi Kaumaha | Hoʻololi Paona (kg) | Pohō hou me Placebo |
|---|---|---|---|
| 5 mg | -15.0% | -16.1 kg | -13.5% |
| 10 mg | -19.5% | -22.2 kg | -18.9% |
| 15 mg | -20.9% | -23.6 kg | -20.1% |
| Placebo | -3.1% | -2.4 kg | — |
Loaʻa ʻo Tirzepatide i ka 15-21% mean o ka hoʻemi kaumaha o ke kino, e hōʻike ana i nā hopena pili i ka dose.
Pākēneka o ka poʻe i komo i ka loaʻa ʻana o ka pohō kaumaha
| Paona Paona (%) | 5 mg | 10 mg | 15 mg | Placebo |
|---|---|---|---|---|
| ≥5% | 85.1% | 88.9% | 90.9% | 34.5% |
| ≥10% | 68.5% | 78.1% | 83.5% | 18.8% |
| ≥15% | 48.0% | 66.6% | 70.6% | 8.8% |
| ≥20% | 30.0% | 50.1% | 56.7% | 3.1% |
| ≥25% | 15.3% | 32.3% | 36.2% | 1.5% |
ʻOi aku ma mua o ka hapaluao na mea komo e loaa ana≥10 mgUa loaʻa iā Tirzepatide≥20% poho kaumaha, e hoʻokokoke ana i ka hopena i ʻike ʻia me ka ʻokiʻoki bariatric.
Nā Pōmaikaʻi Metabolic a Cardiovascular
Hoʻohālikelike ʻia me kahi placebo, ʻoi aku ka maikaʻi o Tirzepatide:
- Poʻai pūhaka
- ʻO ke koko systolic
- ʻIkepili lipid
- Nā pae insulin hoʻokēʻai
Ma waena o nā mea komo meprediabetes, Ua hoʻi ka 95.3% i nā pae glucose maʻamau, hoohalikeia me61.9%i ka hui placebo - e hōʻike ana ʻo Tirzepatide ʻaʻole kōkua wale i ka hoʻohaʻahaʻa kaumaha akā hoʻomaikaʻi pū kekahi i ka metabolism glucose.
Ka palekana a me ka hoʻomanawanui
ʻO nā hopena ʻaoʻao maʻamauʻōpū ʻōpū, menausea, nahu, a me ka constipation, ka hapanui o ka ma'alahi a me ke kuewa.
ʻO ka nui o ka hoʻopau ʻana ma muli o nā hanana ʻino4–7%.
Ua loaʻa kekahi mau make i ka wā o ka hoʻokolokolo, pili nui iCOVID-19, a ʻaʻole pili pono i ka lāʻau haʻawina.
ʻAʻole i ʻike ʻia nā ʻokoʻa koʻikoʻi i nā pilikia pili i ka gallbladder.
Kūkākūkā
ʻO ka hoʻololi ʻana i ke ʻano ola wale nō (ka meaʻai a me ka hoʻoikaika kino) hana maʻamau wale nō~3% awelika poho kaumaha, e like me ka mea i ʻike ʻia ma ka hui placebo.
Ma ka ʻokoʻa, hiki iā Tirzepatide15–21% ka nui o ka hoemi kino, e hoike ana i a5-7 manawa oi ka hopena.
Hoʻohālikelike ʻia me:
- Nā lāʻau hoʻemi kaumaha o ka waha:loaʻa pinepine i ka 5-10% poho
- ʻO ka ʻoki ʻana i ka bariatric:loaʻa iā >20% poho
Hoʻopili ʻo Tirzepatide i ka ʻokoʻa ma waena o nā lāʻau lapaʻau a me nā hana ʻokiʻoki - hāʻawiikaika, hoemi kaumaha ole.
ʻO ka mea nui, ʻaʻole i ʻike ʻia nā hopohopo e pili ana i ka hoʻonui ʻana i ka glucose metabolism. Ma kahi ʻē aʻe, hoʻomaikaʻi ʻo Tirzepatide i ka naʻau o ka insulin a hoʻohuli i ka prediabetes i ka hapa nui o nā mea komo.
Eia naʻe, ua hoʻohālikelike kēia hoʻokolokolo iā Tirzepatide me kahi placebo - ʻaʻole pololei meSemaglutide.
Pono ka hoʻohālikelike poʻo a me ke poʻo no ka hoʻoholo ʻana i ka mea hana e hoʻohua nui i ke kaumaha.
Ka hopena
No nā poʻe mākua me ka momona a i ʻole ke kaumaha a me nā maʻi pili, hoʻohuiʻO Tirzepatide hoʻokahi pulei kahi papahana ola i kūkulu ʻia (ʻai + hoʻoikaika kino) hiki ke alakaʻi i:
- 15-21% awelika ho'ēmi kaumaha kino
- Hoʻomaikaʻi metabolic koʻikoʻi
- ʻO ka tolerability kiʻekiʻe a me ka palekana
No laila ʻo Tirzepatide kahi lāʻau lapaʻau maikaʻi a hoʻopaʻa ʻia i ke kino no ka mālama ʻana i ke kaumaha.
Ka manawa hoʻouna: Oct-16-2025