I kēia mau makahiki i hala iho nei, ua holomua ka holomua o ka mālama ʻana i ka momona a me ka maʻi diabetes type 2. Ma hope o GLP-1 receptor agonists (e laʻa, Semaglutide) a me nā agonists ʻelua (e laʻa, Tirzepatide),Retatrutide(LY3437943), atriple agonist(GLP-1, GIP, a me ka glucagon receptors), ua hōʻike i ka maikaʻi ʻole. Me nā hopena kupaianaha i ka hoʻemi ʻana i ke kaumaha a me ka hoʻomaikaʻi ʻana i ka metabolic, ua manaʻo ʻia ʻo ia ka hopena breakthrough therapy no nā maʻi metabolic.
Mechanism of Action
-
ʻO ka hoʻoulu ʻana o ka mea hoʻokipa GLP-1: Hoʻonui i ka huna ʻana o ka insulin, hoʻopaʻa i ka ʻai, hoʻopaneʻe i ka hemo ʻana o ka ʻōpū.
-
ʻO ka hoʻonā ʻana o ka mea hoʻokipa GIP: Hoʻonui i ka hopena hoʻohaʻahaʻa glucose o GLP-1, hoʻomaikaʻi i ka ʻike insulin.
-
ʻO ka hoʻoulu ʻana o ka mea hoʻokipa ʻo Glucagon: Hoʻoikaika i ka hoʻolilo ikehu a me ka momona metabolism.
ʻO ka synergy o kēia mau mea loaʻa ʻekolu e hiki ai iā Retatrutide ke ʻoi aku ma mua o nā lāʻau i loaʻa i ka pohō kaumaha a me ka mana glycemic.
ʻIkepili hoʻāʻo lāʻau lapaʻau (Phase II)
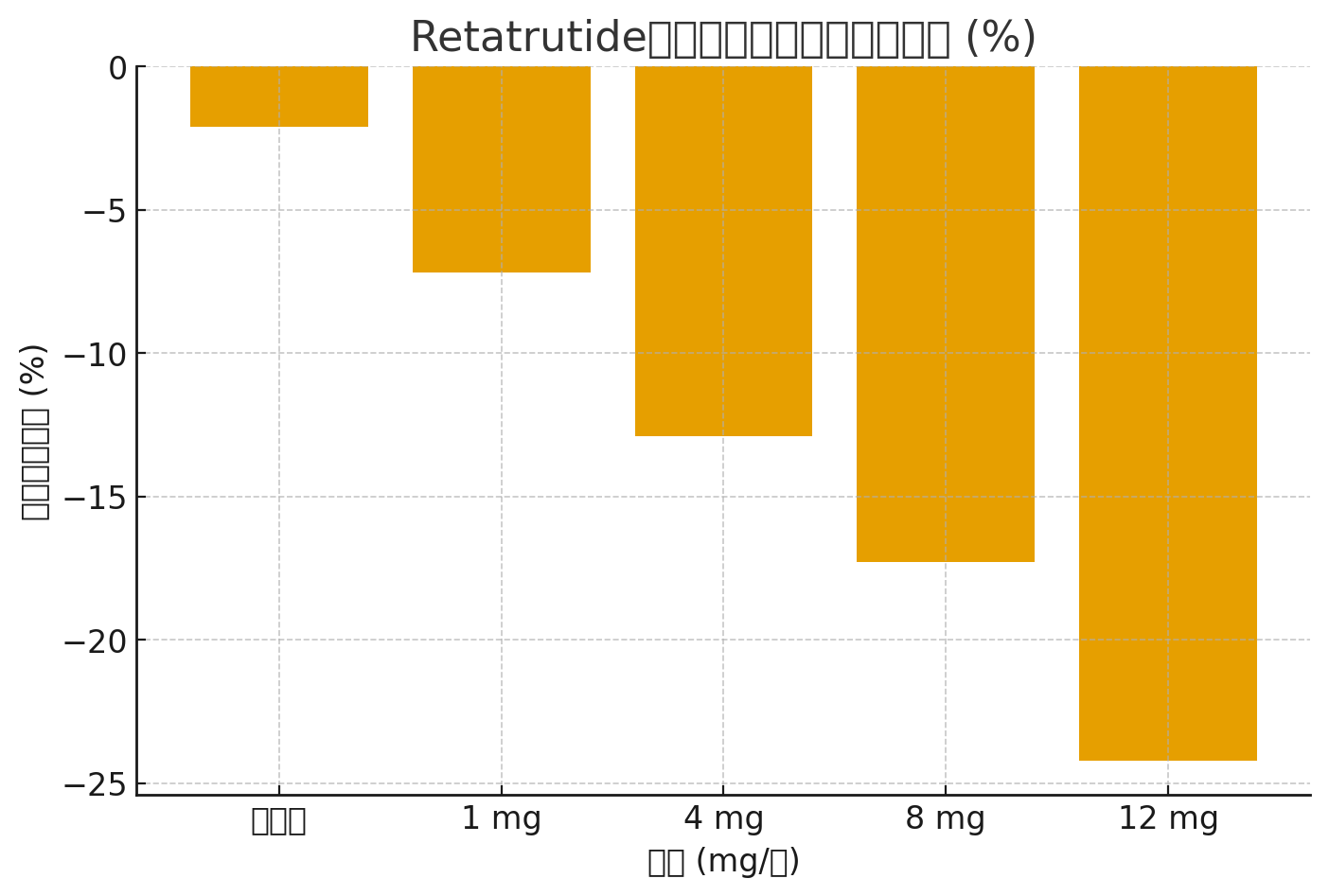
Ma kahiʻO ka hoʻāʻo ʻana o ka Phase II me 338 mau mea maʻi paona / momona, Hōʻike ʻo Retatrutide i nā hopena maikaʻi loa.
Papa: Hoʻohālikelike o Retatrutide vs. Placebo
| ʻAi (mg/wiki) | Mean Weight Hoemi (%) | Hoemi HbA1c (%) | Nā Hana Hoʻopilikia Maʻamau |
|---|---|---|---|
| 1 mg | -7.2% | -0.9% | Nausea, luaʻi mālie |
| 4 mg | -12.9% | -1.5% | Nausea, pau ka makemake |
| 8 mg | -17.3% | -2.0% | ʻAʻole hōʻoluʻolu o GI, ʻaʻohe maʻi maʻi |
| 12 mg | -24.2% | -2.2% | Nausea, pau ka makemake, constipation |
| Placebo | -2.1% | -0.2% | ʻAʻohe loli nui |
Nānā Ikepili (Hoʻohālikelike Hoʻemi Weight)
Hōʻike ka pakuhi pahu ma lalo nei i kahoʻemi kaumaha maʻamauma nā ʻano like ʻole Retatrutide i hoʻohālikelike ʻia me kahi placebo:
Ka manawa hoʻouna: Sep-16-2025